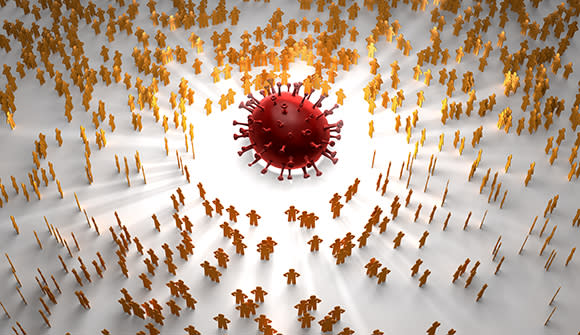
Herd is the word
How herd immunity protects vulnerable people.

How herd immunity protects vulnerable people.
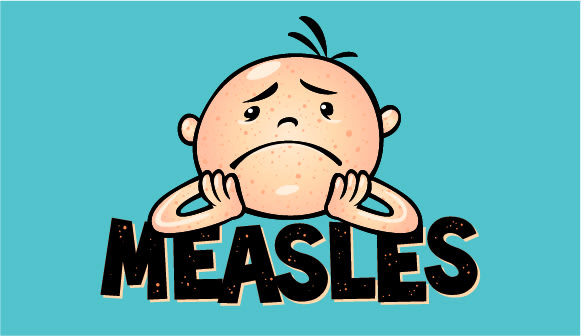
Why the disease is having a resurgence.
How to stay injury-free this holiday season.

The subclade K strain is circulating ahead of the holidays.

Retired agent recovers from bone marrow disease, gifts hope to others.

Use the time to learn about your family’s health history.
Common questions about the virus.

Leave exhaustion at home with these tips to adjust to time differences.
What can we expect this flu season?